Establishment of blood-brain barrier as a novel therapeutic and diagnostic target
~Brain Barrier System as a Target for Diagnosis and Drug Discovery~
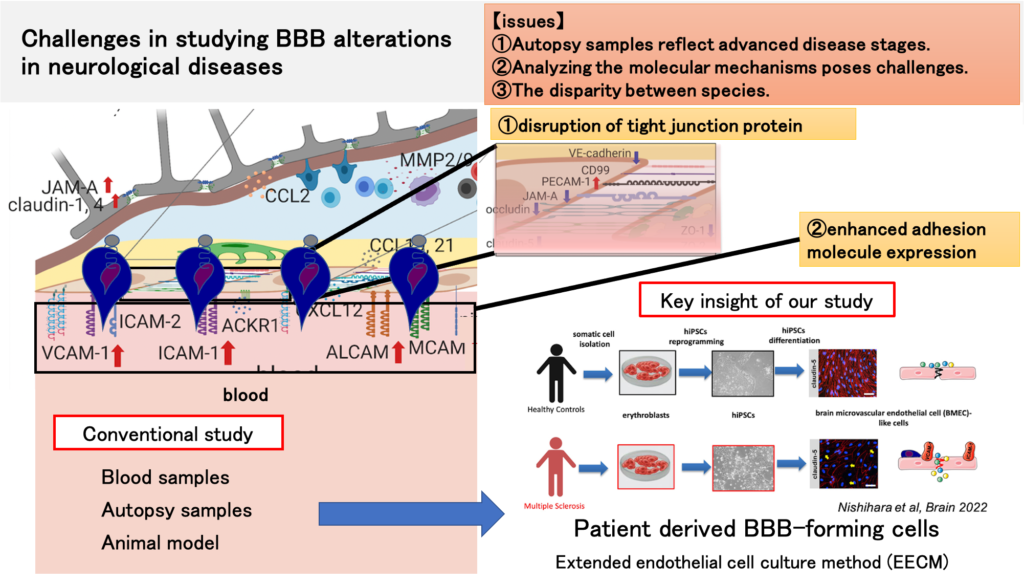
Pathological studies show blood-brain barrier (BBB) disruption in many neurological diseases, however, due to the limited access to disease-related BBB samples, it still is not well understood, if BBB malfunction is causative for disease development or rather a consequence of neuroinflammatory or neurodegenerative process. Recent stem cell technology provides the opportunity to establish in vitro BBB models from patients and thus to study disease-specific BBB characteristics. We have established a novel human induced pluripotent stem cells (hiPSCs)-derived BBB model, which resembles primary human brain microvascular endothelial cells in morphology, junctional architecture, and diffusion barrier characteristics. Proper endothelial adhesion molecule expression makes this model distinct from any other hiPSC-derived BBB model and suitable to study immune cell interaction with the BBB. Importantly, our models can mimic BBB dysfunction in multiple sclerosis, thus useful to study disease pathomechanisms. We aim to model BBB dysfunction in several neurological diseases. We derivate BBB-forming cells including endothelial cells, mural cells, and astrocytes from hiPSCs. This model allows us to analyze the function of the BBB, therefore we anticipate answering how BBB dysfunction contributes to disease pathomechanisms. Furthermore, we plan to establish diagnostic markers for BBB disruption and to identify novel therapeutic targets for BBB stabilization.
Summary of Research Results to Date.
Elucidating the Mechanisms of CNS Barrier System Failure.
We are actively investigating the mechanisms behind the disruption of the blood-brain barrier (BBB) in neurological diseases. Our approach involves utilizing an immortalized human in vitro BBB model, serum and immune cells samples obtained from patients with neurological diseases. A recent milestone in our research is the development of an original patient-derived BBB model derived from induced pluripotent stem (iPS) cells. This innovative model (FASEBJ2020), for which we hold an international patent, enables us to uncover the pathomechanisms underlying neurological diseases. Notably, this model successfully replicates BBB disruption observed in the autopsy brains of multiple sclerosis patients (Barin 2022). Moreover, our method proves to be valuable for evaluating BBB function and is an effective tool for drug screening aimed at stabilizing the BBB.
Future Research Plans
We aim to advance innovative research by employing stem cell technology to induce the differentiation of patient-derived blood-brain barrier (BBB) constituent cells. Our overarching goal is to elucidate the mechanisms underlying BBB breakdown for the development of biomarkers and pharmacological interventions targeting BBB dysfunction, ultimately reaching clinical applications. Our research focuses on the following themes:
1. Analysis of the Impact of BBB Breakdown on the Clinical Presentation of Multiple Sclerosis Patients:
Multiple sclerosis is a heterogeneous disease with various subtypes, and the reasons for individual differences remain unknown. We have proposed a challenging hypothesis that the genetic vulnerability of the BBB plays a pivotal role in the progression of symptoms, and our research aims to explore this hypothesis.
2. Elucidating the Mechanisms of BBB Breakdown in Cerebral Small Vessel Disease:
Focusing on hereditary cerebral small vessel disease, we create BBB constituent cells from patient samples to unravel the mechanisms of BBB breakdown. Recent findings reveal that genes previously considered rare in hereditary cerebral small vessel disease may become risk genes for sporadic cerebral small vessel disease, such as lacunar infarctions. Using insights gained from understanding hereditary cerebral small vessel disease, we aspire to contribute to the resolution of more common conditions like lacunar infarctions and vascular dementia.
3. Understanding the Mechanisms of BBB Breakdown in Neurodegenerative Disorders and Investigating the Link between Peripheral Inflammation and Central Nervous System Degeneration:
Creating BBB models from patients with neurodegenerative disorders such as Alzheimer’s, Parkinson’s, amyotrophic lateral sclerosis, and Huntington’s diseases, we conduct research to elucidate “common” or “disease-specific” mechanisms of BBB breakdown. Additionally, we investigate how peripheral inflammation, a recently highlighted factor, contributes to central nervous system degeneration.
4. Enhancing BBB Model Construction:
Acknowledging the absence of a perfect in vitro BBB model reflecting all in vivo functions, we emphasize the importance of selecting an optimal model based on individual research objectives. Our studies focus on improving our unique model, which has advantages in endothelial cell morphology, transcriptome expression, and immune cell interactions. Specific enhancements include the creation of a 3D BBB model, the development of co-culture models with central nervous system constituent cells, and refining endothelial cells’ BBB characteristics through small-molecule treatments.
